Future of Cell & Gene Therapy Market | Top Trends, Innovations, and Key Regional Developments | DataM Intelligence
A complete guide to Cell & Gene Therapy Market growth key players, latest breakthroughs, regional trends & market forecasts through 2033.
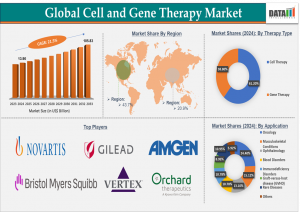
Cell & Gene Therapy surging to US$105.83B by 2033, growing 21.5% annually reshaping treatment for cancer, rare diseases & chronic conditions.”
AUSTIN, TX, UNITED STATES, June 20, 2025 /EINPresswire.com/ -- The Cell and Gene Therapy Market reached US$ 13.90 billion in 2024 and is projected to surge to US$ 105.83 billion by 2033, growing at a remarkable CAGR of 21.5% during the forecast period 2025-2033. This extraordinary pace reflects the rapid advancements in biotechnology, increasing global demand for personalized therapies, and breakthroughs in genetic engineering and regenerative medicine.— DataM Intelligence
Cell and gene therapies are transforming the treatment landscape for a range of conditions from rare genetic disorders to widespread chronic diseases like cancer. With therapies now making their way from research labs into real-world clinical use, the market is entering an exciting period of accelerated commercialization.
To Download Sample Report: https://www.datamintelligence.com/download-sample/cell-and-gene-therapy-market
Key Growth Drivers
Rising Demand for Personalized Medicine
There is a growing global shift toward personalized and precision medicine, where treatments are tailored to an individual’s genetic makeup and disease profile. Cell and gene therapies lie at the heart of this transformation, offering customized solutions for complex and previously untreatable conditions. From CAR-T cell therapies that target specific cancer mutations to gene therapies correcting rare inherited disorders, the ability to create patient-specific treatments is driving strong demand across both developed and emerging healthcare markets.
Increased Investment in R&D
Both public and private sector investments in CGT are accelerating at an unprecedented pace. Major pharmaceutical companies, biotechnology firms, venture capital groups, and government initiatives are collectively pouring billions of dollars into expanding CGT pipelines. This surge in funding is fueling a sharp increase in the number of clinical trials, helping therapies advance from early-stage research to late-stage development and commercialization. The availability of capital is also supporting innovation in next-generation technologies such as gene editing and cell reprogramming.
Recent Developments in Cell and Gene Therapy Market
In September 2024, CPC (Colder Products Company), a Dover company and a leading provider of connection technologies for biopharmaceutical processing, introduced a new aseptic micro-connector designed to integrate directly with freeze cassettes used in cell and gene therapy (CGT) workflows.
In April 2024, Walgreens announced plans to collaborate directly with drug manufacturers to expand patient access to CGTs in the U.S. This initiative is part of a broader enhancement of its specialty pharmacy services, which now include a new business unit dedicated to specialty pharmacy incorporating its AllianceRx subsidiary.
In May 2024, ProPharma, a U.S.-based regulatory, clinical, and compliance service provider (part of Odyssey Investment Partners), partnered with Italy’s PBL to launch the Cell Factory Box (CF Box) an enclosed, fully automated device that supports decentralized manufacturing of various CGTs in Class D (ISO8) or controlled environments.
Regional Outlook
North America
North America continues to dominate the global CGT market, thanks to a strong ecosystem of biotech firms, robust clinical trial activity, and favorable regulatory pathways. The U.S. alone accounts for a significant share of global revenues, with major hubs in Boston, San Francisco, and North Carolina driving innovation.
Government support for breakthrough therapies, along with strong public-private partnerships, has positioned the U.S. as a leader in the development and commercialization of CGTs.
Europe
Europe remains an important player in the CGT space, driven by countries such as Germany, the UK, France, and the Netherlands. Recent regulatory reforms and cross-border partnerships are accelerating the approval and delivery of new therapies across the continent.
Asia-Pacific
Asia-Pacific, and especially Japan, is emerging as one of the fastest-growing regions in the CGT market. Government policies supporting regenerative medicine, combined with advanced manufacturing capabilities, are helping to scale up commercial production and broaden access to therapies.
China and South Korea are also investing heavily in gene therapy platforms, further boosting the regional market potential.
Market Segmentation
By Therapy Type: Cell Therapy, Gene Therapy.
By Application: Oncology, Musculoskeletal Conditions, Ophthalmology, Blood Disorders, Immunodeficiency Disorders, Graft-versus-host disease (GVHD), Rare Diseases, Others.
By Region: North America, Latin America, Europe, Asia Pacific, Middle East and Africa.
Major Companies in Cell and Gene Therapy Market
Novartis AG
Gilead Sciences, Inc.
Bristol Myers Squibb Company
Vertex Pharmaceuticals Incorporated
Sarepta Therapeutics, Inc.
CSL Behring LLC
Amgen, Inc.
Orchard Therapeutics group.
Krystal Biotech, Inc.
bluebird bio, Inc.
Latest News of USA
The U.S. CGT market is advancing rapidly, highlighted by a recent US$1 billion+ acquisition of a gene-editing biotech, underscoring the growing importance of gene therapies for cardiovascular and chronic diseases. At the same time, the industry faces challenges, as safety concerns surrounding a prominent DMD gene therapy led to trial suspensions and FDA reviews. Despite this, innovation continues, with a groundbreaking in vivo CRISPR therapy successfully used to treat a baby with a rare metabolic disorder—showcasing the promise of next-gen pediatric gene therapies.
Latest News of Japan
Japan is making swift progress in CGT development, with the Ministry of Health, Labour and Welfare recently granting conditional approval for a DMD gene therapy, reflecting strong regulatory support for innovative treatments. At the same time, the country is investing heavily in smart manufacturing including a robotics- and AI-powered cell therapy plant in Chiba, set to open in 2026 and scaling up AI- and blockchain-enabled cold-chain logistics to ensure safe, efficient delivery of advanced therapies across its healthcare system.
Conclusion
The global Cell and Gene Therapy (CGT) market is evolving at an extraordinary pace, fueled by scientific innovation, increasing investment, and the rising demand for personalized treatments. While North America continues to lead, Japan and the broader Asia-Pacific region are quickly emerging as key growth hubs.
As the market advances, both opportunities and challenges will shape its trajectory. Strategic partnerships, advanced manufacturing, and strong safety frameworks will be essential to sustaining momentum and ensuring broad patient access. From groundbreaking gene editing to next-generation cell therapies, CGTs are set to transform the future of healthcare worldwide.
Looking For A Detailed Full Report? Get it here: https://www.datamintelligence.com/buy-now-page?report=cell-and-gene-therapy-market
Purchase Your Subscription to Power Your Strategy with Precision: https://www.datamintelligence.com/reports-subscription
Related Reports:
Cell and Gene Therapy Manufacturing Services Market
Gene Editing Tools Market
Sai Kumar
DataM Intelligence 4market Research LLP
+1 877-441-4866
email us here
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.